Barco MDRC-1219 19" Clinical Review Display Monitor
Barco MDRC-1219 Eonis 19-inch Clinical Review Display Monitor
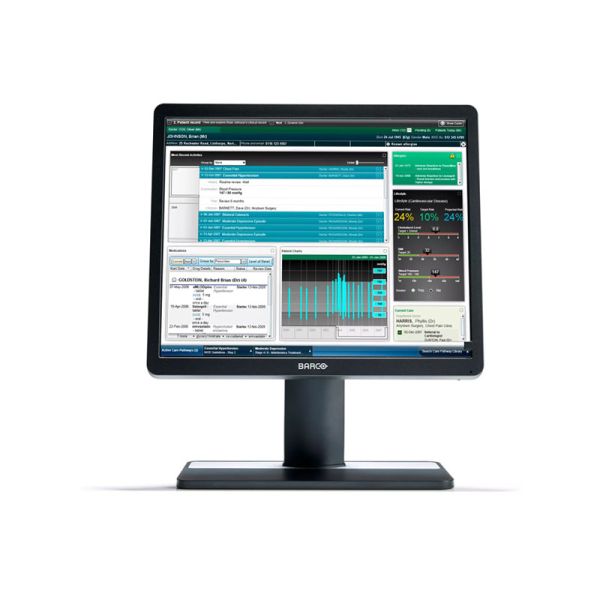
- Eonis 19" clinical display for hospital-wide viewing of clinical data and images
- Fit for clinical use
- Front consistency sensor technology
- Quality assured, automatically
- Ampronix is a Authorized Barco Reseller
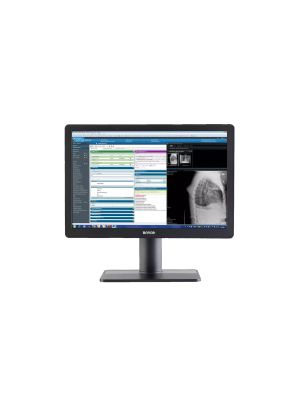
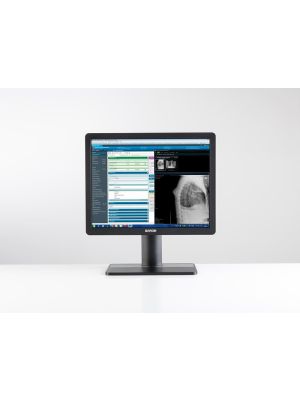
The Barco MDRC-1219 Eonis 19" brings reliable, DICOM compliant images to a broad palette of imaging applications, including EMR. It combines consistent image quality and an attractive, versatile design with effortless quality assurance. A touchscreen version is also available.
The high-quality display presents sharp, bright images with high contrast. The unique front consistency sensor automatically aligns the image quality every time the display is switched on, ensuring fast power-up and continuous luminance stability.
Result? Consistent images that make collaboration between specialists easier. They can discuss images with colleagues at multiple locations, knowing that everyone is seeing identical images.
The MDRC-1219 comes with Barco’s cloud-based MediCal QAWeb software, an online service for calibration, Quality Assurance, and asset management. Praised in hospitals around the world, MediCal QAWeb allows healthcare IT and PACS administrators to centrally and remotely manage image quality across the healthcare organization, at the click of a button.
The unique 5:4 aspect ratio is ideal for viewing all types of applications and images from medical modalities. Like every Barco clinical display, the MDRC-1219 complies with international medical and patient safety standards.
Part Number: K9301820A, Model: MDRC-1219
Part Number: K9301821A, Model: MDRC-1219 (option TS)
Part Number: K9350030, Model: MDRC-1219 (option HB)
Part Number: K9350281, Model: MDRC-1219 HB
Part Number: K9350031, Model: MDRC-1219 (option HB TS)
| Manufacturer | Barco |
|---|---|
| Condition | New |
| Screen Technology/Type | Standard versions: IPS TFT, High bright versions: IPS AHVA |
| Screen Size (diagonal) | 482 mm (19.0") |
| Display Resolution | 1MP (1280 x 1024 pixels) |
| Pixel Pitch | 0.294 mm |
| Viewing Angle (H, V) | 178° |
| Maximum Luminance | Standard versions: 330 cd/m², High bright versions: 700 cd/m² |
| Country of Manufacture | Italy |
| Contrast Ratio | Standard versions: 1000:1, High bright versions: 900:1 |
| Response Time | Standard versions: 15 ms, High bright versions: 12.5 ms |
| Video Input Connectors | DVI (DVI-D and DVI-A) DisplayPort |
| Power Input Requirements | Internal power supply (100-240 Vac, 50/60 Hz) |
| Power Consumption | Standard versions: 22 W (nominal), High bright versions: 25 W (nominal) |
| Power Save Mode | < 0.5 W (standby) |
| Dimensions with Stand (W x H x D) | Portrait: 348 x 440~550 x 201 mm, Landscape:411 x 408~518 x 201 mm |
| Dimensions without Stand (W x H x D) | 411 x 348 x 67 mm |
| Net Weight with Stand | MDRC-1219: 5.3 kg, MDRC-1219 (option TS): 6.1 kg, MDRC-1219 (option HB): 5.4 kg, MDRC-1219 (option HB TS): 6.2 kg |
| Net Weight without Stand | MDRC-1219: 3.1 kg, MDRC-1219 (option TS): 3.9 kg, MDRC-1219 (option HB): 3.2 kg, MDRC-1219 (option HB TS): 4.0 kg |
| Mounting Options | VESA (100 mm) |
| Certifications | Standard versions: FDA Class I,510(k) exempt CE (Medical Device Class I) CCC (China), Inmetro (Brazil), KC (Korea), BIS (India), EAC (Russia, Kazakhstan, Belarus, Armenia and Kyrgyzstan) |
| Supplied Accessories | 1x DisplayPort video cable 1x USB cable 1x printed User Guide (English) 1x system disc, containing QAWeb software and translations of the User Guide Mains cables |
| Warranty | 3 years |
| Modality | Clinical Review |